Your expensive marine sensor vanishes beneath the waves for a year-long mission. A battery failure means more than lost data; it means a lost asset and a wasted budget.
Lithium Manganese Dioxide (Li-MnO₂) batteries1 excel in marine environments due to their unmatched reliability. Their very low self-discharge, wide operating temperature range, and stable, long-life power output make them the default choice for "deploy and forget" applications like buoys and subsea sensors.

I've spent years helping clients who work in oceanography. Their equipment has to survive incredible pressures and extreme temperatures for months or even years without intervention. For them, a battery isn't just a component; it's the lifeline of their entire project. Rechargeable batteries introduce too many failure points. For these critical applications, the proven reliability of a primary battery is essential. The "charge-free, stable output" of Li-MnO₂ is precisely what these scenarios demand, making it a technology trusted by marine instrument manufacturers2 worldwide.
Do lithium manganese dioxide batteries self discharge?
You're designing a device that needs to sit on a shelf for a year, then be deployed for another two. You worry that any normal battery would be dead before it even sees the water.
Yes, but at an incredibly slow rate. Li-MnO₂ batteries have a very low self-discharge rate, typically less than 1% per year. This makes them perfect for long-term deployments where reliability is everything.
Self-discharge is the silent mission killer. For a device that's unattended for years, even a tiny energy leak can drain the battery completely. The magic of Li-MnO₂ lies in its stable chemistry and robust construction. The materials—a solid manganese dioxide cathode and a lithium metal anode—are inherently stable and don't react much when the battery isn't being used. This is completely different from rechargeable lithium-ion batteries3, which can lose several percent of their charge every month. We seal our Li-MnO₂ cells with a glass-to-metal hermetic seal. This technology, borrowed from military and aerospace applications, creates a true physical barrier that stops microscopic leaks, ensuring the power we pack inside stays there for a decade or more.
| Battery Type | Typical Self-Discharge Rate | Ideal Use Case |
|---|---|---|
| Li-MnO₂ | < 1% per year | Long-term, low-power (e.g., ADCP) |
| Alkaline | 2-3% per year | General consumer electronics |
| Li-ion (NMC) | 2-5% per month | High-drain, rechargeable (e.g., laptop) |
How do lithium manganese dioxide batteries work?
You know they are reliable, but as an engineer, "it just works" isn't enough. You need to understand the basic principle to confidently design it into your system.
A Li-MnO₂ battery generates power through a simple, one-way chemical reaction. It has a lithium metal anode (the negative terminal) and a solid manganese dioxide cathode (the positive terminal). When connected, lithium ions flow to create a very stable 3.0V electrical current.
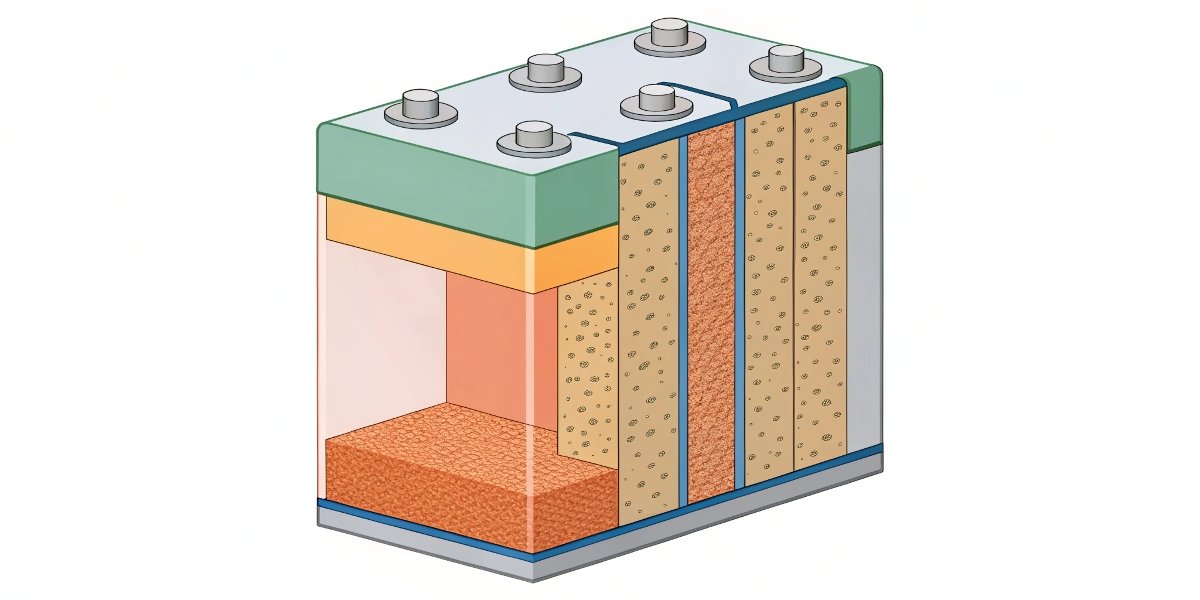
The beauty is in the simplicity. There are no complex liquid electrolytes or chemistries that need constant monitoring like in rechargeable batteries. We construct these cells in two main ways: a "bobbin" type, which packs the materials tightly to maximize energy for low-power devices, or a "spiral" type, which has more surface area for higher power pulses. Most importantly for marine use, the battery is hermetically sealed. This robust, welded steel can with glass-to-metal seals prevents any water from getting in, even under deep-sea pressure. This means our Li-MnO₂ batteries can often be used as the primary power source without needing a separate, bulky waterproof enclosure. It simplifies your product's design, reduces weight, and is one less thing that can fail.
What are the problems with lithium batteries in boats?
You constantly hear about the fire risks of "lithium batteries," making you nervous about putting them on a boat or, worse, inside a sealed underwater pod where failure is catastrophic.
The most cited problems, like fire and thermal runaway, are linked to rechargeable lithium-ion chemistries (like NMC), not stable primary cells. The risk comes from overcharging, physical damage, or a failure in the complex charging electronics, none of which apply to Li-MnO₂.
[^4] circuit board](https://lithotop.com/wp-content/uploads/2025/07/4-the-most-cited-problems-like-fire-and-thermal-r.jpg)
It's critical to understand that "lithium battery" is a very broad term. The battery in your laptop (NMC or a similar Li-ion chemistry) is designed for high power and hundreds of recharge cycles. Its disadvantage is that it contains a lot of energy in a less stable state. It absolutely requires a sophisticated Battery Management System (BMS) to protect against over-charging, over-discharging, and overheating. Any failure in the battery or the BMS can lead to a dangerous chain reaction. In contrast, a primary Li-MnO₂ battery has a much safer, more stable chemistry. It's designed for one-time discharge. There is no charging. This removes the single biggest source of risk. For marine devices needing high power pulses, like an acoustic pinger, we create a hybrid solution. We pair a Li-MnO₂ battery with a capacitor, providing the best of both worlds: the capacitor delivers the high pulse, while the battery provides decades of safe, reliable standby power.
What is the disadvantage of an NMC battery?
You see NMC batteries used in everything from power tools to electric vehicles. You wonder why this popular, high-energy chemistry isn't the go-to solution for everything, including marine sensors.
The main disadvantage of an NMC (Lithium Nickel Manganese Cobalt Oxide) battery is its complexity and lower stability compared to primary lithium cells. It requires a sophisticated BMS to operate safely, has a higher self-discharge rate, and is more susceptible to thermal runaway.
NMC batteries are fantastic for their intended purpose: storing a lot of energy in a small, lightweight, and rechargeable package. This is perfect for a drone or an e-bike where you need high power and can recharge it every day. However, these same strengths become weaknesses in a long-term, unattended device. The high energy density comes with higher risk. The need for a BMS adds another layer of electronics that can fail. The higher self-discharge means it can't sit for years and be expected to work. In the harsh, inaccessible marine environment, simplicity equals reliability. You want the safest, most stable chemistry possible. The lower energy density of a primary cell like Li-MnO₂ is a worthwhile trade-off for its incredible long-term stability and inherent safety, which are the most important features for a product manager designing a system for a deep-sea deployment.
Conclusion
For marine applications, choose safety and reliability. Li-MnO₂ batteries provide stable, long-life power for years, eliminating the risks and complexity of rechargeable options. They are the proven solution for your mission-critical equipment.




